Bone Density Screening Made Easy
Start improving your measures with our bone density scanner – Now 3 Times Faster Patient Screening
01
Portable
02
Fast
03
Report
Start Improving Your Measures Now
BeamMed’s HEDIS OMW Bone Density Solution that fit the NCQA HEDIS measures and close the HEDIS gaps.
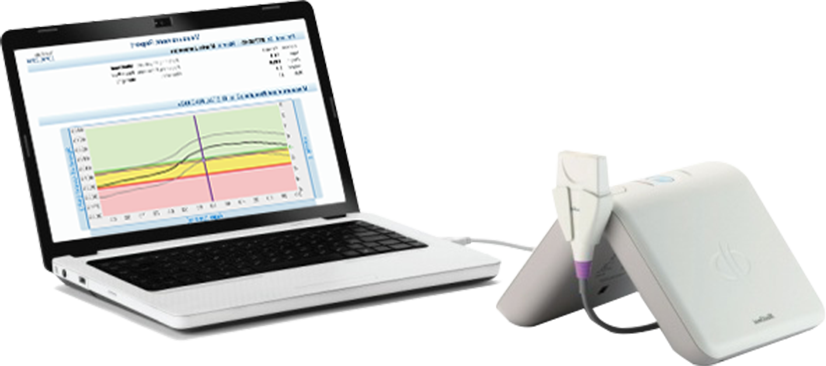
Sunlight MiniOmni™ is an ultra-small screening device that can bring a huge benefit to osteoporosis diagnosis and monitoring. Highly affordable and easy to use, it delivers accurate bone density assessments. Click Play to see how Sunlight MiniOmni enables early assessment of osteoporosis – without x-rays. Ultra-small and light bone sonometer that enables reliable, accurate, non invasive and safe early assessment and monitoring of bone density – with exceptional cost-effectiveness. USB connectivity to PC/laptop.

Upcoming Events
You are cordially invited to join us at any future events. Click below to view our latest upcoming events.
Qualipalooza: RISE Quality Leadership Summit 2024
Signia Hilton
159 Northside Dr NW, Atlanta, GA 30313
RISE West Summit Annual 2024, Colorado Springs CO
The Broadmoor
1 Lake Ave, Colorado Springs, CO 80906
HEDIS & Quality Improvement Summit 2024
Luminary Hotel & Co.
2200 Edwards Dr, Fort Myers, FL 33901
RISE CMS Star Ratings Master Class 2024
InterContinental hotel San Diego
InterContinental San Diego, an IHG Hotel, Bayfront Court, San Diego, CA, USA

MiniOmni
Portable. Affordable.
For Portable Bone Density Solutions, Call (800) 769-6808
Affordable, Professional Solutions for Early Assessment of Osteoporosis.